Global coup for CMR Surgical as FDA backs Versius robot surgery for US market
The FDA has approved the company’s surgical robot Versius initially for gallbladder removal – a big enough market in its own right as the eighth most common operating room procedure performed in hospitals in the US – but CMR Surgical is confident that this is just the start.
Named International Trade Champion in the recent Business Weekly Awards, CMR Surgical’s arrival in American hospitals really shakes up that whole, highly lucrative marketplace.
Until now, the US has performed the world’s most operations using robots and has the richest, most significant manufacturers on the planet in this rarefied field of technology.
CMR Surgical, a long-term Cambridge unicorn, now has the opportunity to grow exponentially on the back of a transatlantic boom as authorisation for the Versius Surgical System paves the way for a nextgen, adaptable and digitally driven surgical robot to be introduced the length and breadth of the US.
Versius is the first multi-port, soft tissue general surgical Robotic Assisted Surgical Device (RASD) to be backed through FDA’s De Novo application process.
Globally, Versius is the second most used surgical robotic system with over 26,000 surgical procedures completed in seven specialties as approved in those markets.
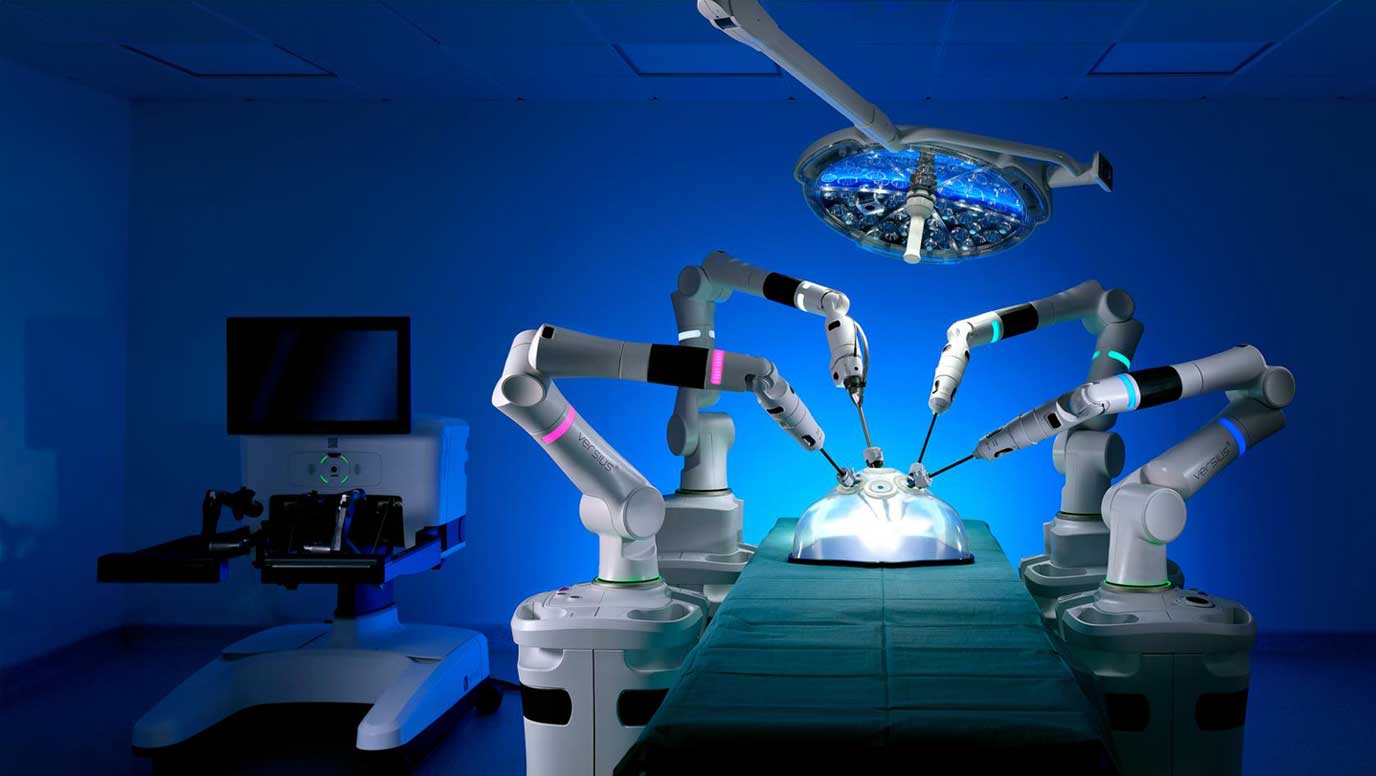
Versius is compact and modular, making it a versatile and portable surgical robot that can seamlessly integrate into virtually any operating room to perform cholecystectomy procedures. It can be easily moved between departments, making it suitable for any care setting.
Versius is designed to biomimic the human arm, empowering surgeons through optimised port placement together with the dexterity and accuracy of small, fully-wristed instruments.
It offers 3D HD vision, easy-to-adopt instrument control and a choice of ergonomic working positions with an open surgeon console that has the potential to reduce stress and fatigue while allowing for clear communication with the surgical team.
Mark Slack, Chief Medical Officer and Co-founder at CMR Surgical, says: “Securing FDA marketing authorisation for Versius for use in cholecystectomy in adult patients is a significant milestone for CMR and, most importantly, for hospitals and patients who will now have greater access to robotic-assisted surgery in cholecystectomy procedures.
“With FDA authorisation, we can now bring our compact and portable surgical robot to the world’s largest healthcare market, expanding the benefits of robotic-assisted surgery with Versius across various care settings in the US.”
As a digitally driven system, Versius is rooted in continuous innovation driven through software to ensure customers benefit from the latest technology updates.
With a suite of digital apps to support surgeons, surgical teams and hospitals, Versius provides real time insights to improve proficiency and optimise surgical robotic programs.
As CMR brings Versius to customers across the US, the Company will also roll out its state-of-the art global metrics-based training program. The training pathway draws on cutting-edge technology including Versius Trainer and the Versius eLearning platform, as well as peer to peer education through preceptoring and ongoing guidance and support, ensuring smooth transition from training to the operating room.CMR’s training pathway also includes Versius Virtual Reality and Versius Trainer in VR improving training pathway accessibility.
Massimiliano Colella, Interim Chief Executive Officer at CMR Surgical, commented: “The US is an important strategic market so gaining FDA authorisation for Versius for use in cholecystectomy procedures in adult patients is a significant step forward in CMR achieving its mission of bringing minimal access surgery to more patients around the world.
“The value of Versius as a compact and modular system has been demonstrated by leading hospitals around the world and we look forward to working closely with hospitals in the US to introduce Versius.”
The scale of the opportunity for CMR was explained by Erik Wilson, M.D., Division Director and Vice Chair of Surgery at University of Texas McGovern Medical School at Houston.
He said: “I have enjoyed being part of the development of Versius since 2017 and it is very exciting that hospitals across the US can benefit from this next-generation technology.
“The US is a mature market for surgical robots so it is hard to believe that until today there was little choice when deciding to start a soft tissue robotics program. Versius securing FDA clearance is an important step forward for helping hospitals of any size to be able to offer robotic-assisted surgery.”
The US is an important market with a strong demand for a flexible surgical robotic system that can be used across any care setting. Of nearly 10 million annual major operating room procedures in the US, only around 2.5 per cent were robotic assisted.
Versius could enable patients to undergo minimal access surgery for cholecystectomy procedures closer to home thanks to this compact, modular, portable system.
The FDA authorisation is the first part of a multi-stage strategic plan that will involve initially partnering with a select number of US hospitals, putting CMR and Versius in a strong position to enter the American market and expand its partnership with hospitals across the country.
Steven D. Schwaitzberg, M.D., Chairman of the Department of Surgery at University at Buffalo School of Medicine and Biomedical Sciences, said: “For more than a decade there has been a rapid expansion of procedures performed with robotic assistance.
“There has not been enough choice in the surgical arena for robotic systems in the US. I am delighted that Versius is entering into the marketplace with FDA marketing authorisation.
“There is a real need for more compact, modular systems that can be adopted for care in a variety of settings. I look forward to exploring these new developments in robotic assisted surgery as Versius comes to the US.”
Robert Tansley, Partner with investor, Cambridge Innovation Capital, was thrilled by the development. He said: “Versius is currently approved in over 40 countries worldwide and is commercially available in more than 30.
“The FDA marketing authorisation represents a pivotal achievement for CMR Surgical, unlocking access to the US market – the largest healthcare market in the world. The US breakthrough signals a significant growth potential for CMR, creating a pathway for broader adoption of minimal access surgery and driving long-term value for stakeholders.”