ClariMed opens research facility in Cambridge
The company has its headquarters in Pennsylvania and other US facilities in California (San Jose) and Cambridge, MA. It has now opened an advanced simulated use research facility at Copley Hill Business Park, Babraham.
ClariMed says the strategic investment significantly expands its capacity to host and conduct research to support user-centered medical device development.
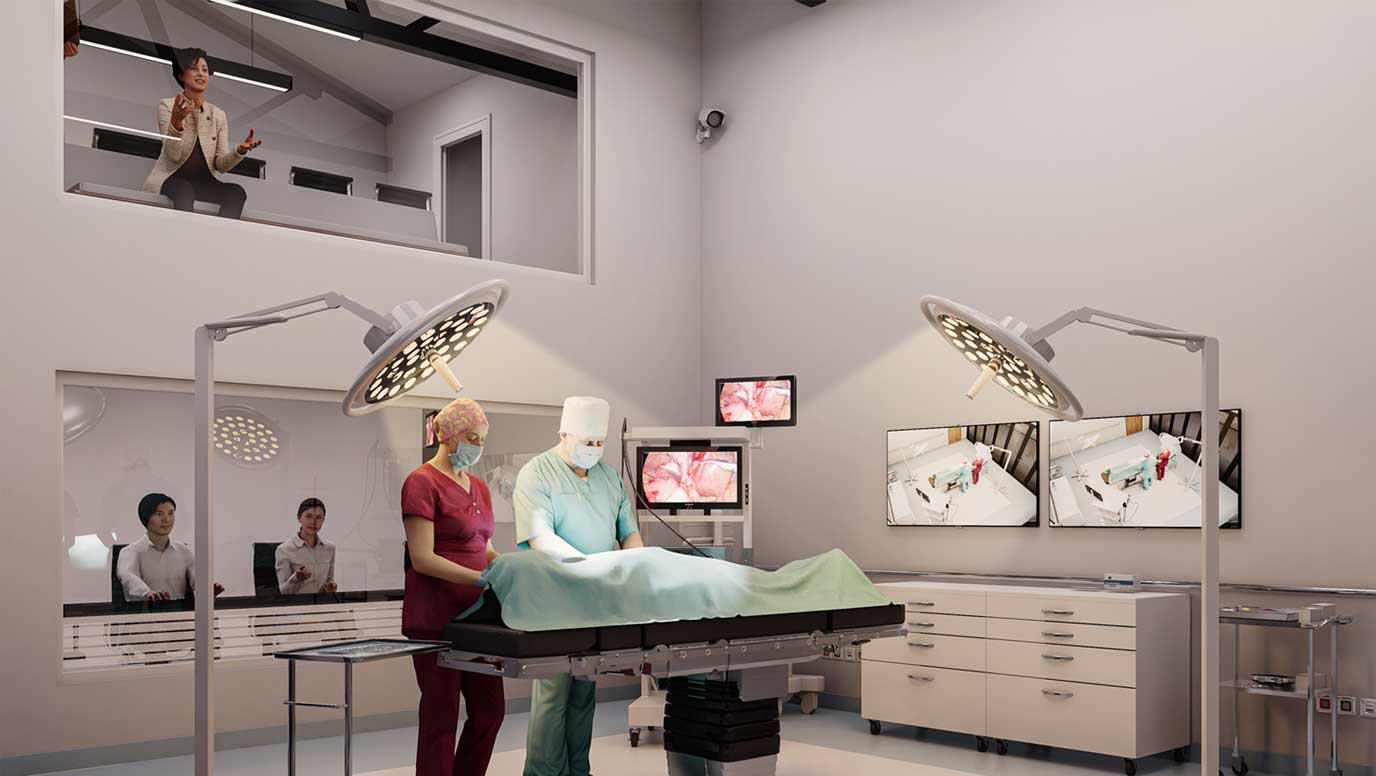
The purpose-built facility features innovative design elements that enhance the quality and scope of human factors studies, including a dedicated second-floor observation area that provides unparalleled visibility of participant interactions. This setup enables clients and researchers to observe studies with minimal interference, ensuring more natural user behaviours and more accurate research outcomes.
Julian Dixon, Associate Director of Human Factors and Site Lead of the Cambridge, UK office, says: “This facility represents a significant advancement in our ability to conduct sophisticated usability studies.
“The elevated observation area offers our clients unprecedented visibility into user interactions, while our enhanced recording capabilities and flexible study spaces allowing us to simulate various usage environments with exceptional fidelity.”
Kelley Kendle, CEO of ClariMed, emphasised the strategic importance of the expansion: “This investment significantly enhances our ability to serve global clients with increasingly complex research needs. The advanced capabilities of our new facilities enable us to conduct more sophisticated studies, ultimately leading to safer and more effective medical devices.”
The facility has been designed to support a wide range of human factors studies, from early-stage concept testing to validation studies. Its versatile configuration allows for evaluating everything from handheld devices to surgical robots, with specialised areas for home-use simulation and professional healthcare environments.
ClariMed is a human-centered development and regulatory practice for medical products developed by pharmaceutical and medical device manufacturers.

